Feb 5, 2018
LDH cytotoxicity check for continuous batch control
Our daily routine ensures that you are receiving only Phenion products which fulfill our high-quality standards. Starting with the human biopsy material, continuing with the isolated primary cells and ending with the fully differentiated skin equivalents, the production processes are carefully monitored to ensure that our pre-defined specifications are completely met.
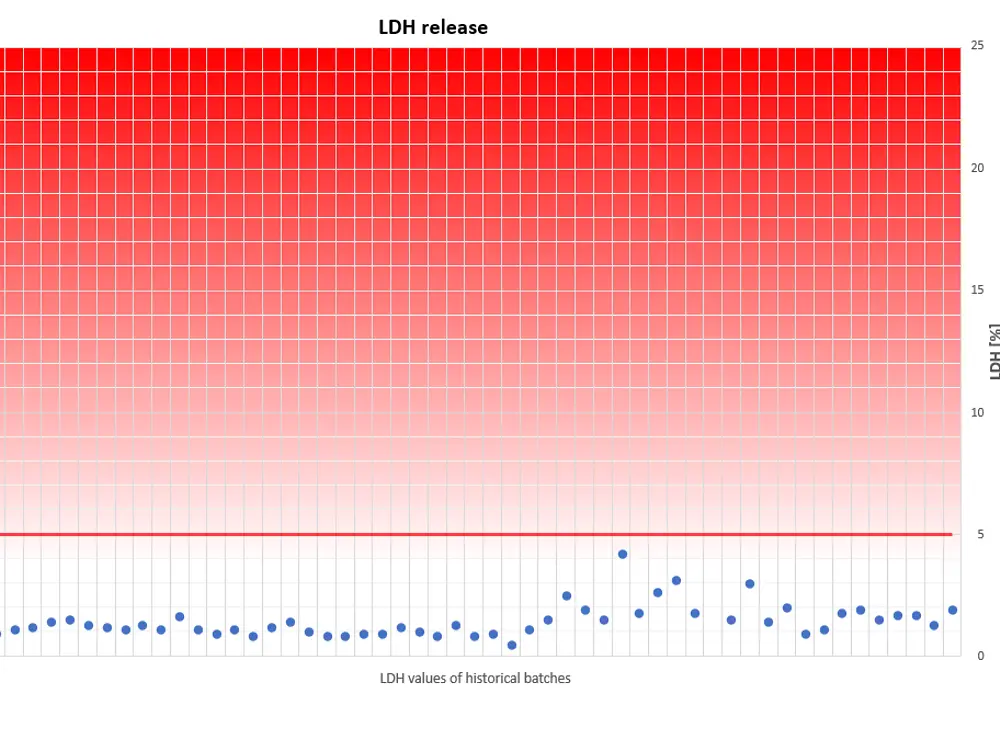
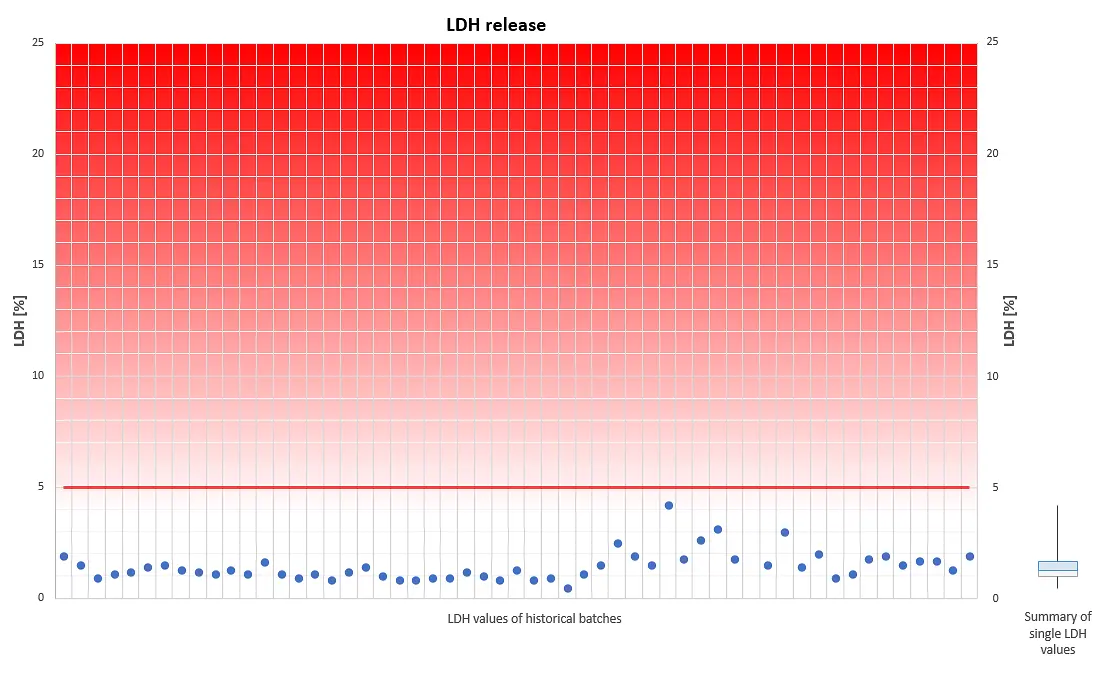
The viability of our fully differentiated skin models is routinely monitored with the Lactate dehydrogenase (LDH) assay. Lactate dehydrogenase, an intracellular enzyme, can leak out of the cells only when the cell membranes are damaged, e.g. due to unexpected effects which consequently can reduce cell viability. Thus, LDH release from intact skin models should be minimal when cultured under our standardized conditions. LDH can be easily detected and quantified in the cell culture medium.
A skin model production batch is qualified for shipment only when the amount of LDH, which leaks from individual tissues into the cell culture medium within 24 hours, is less than 5% of the LDH amount which is released within the same period by TRITON X-100- treated skin models.
The diagram visualizes a compilation of historical LDH data. It reveals that LDH leakage never exceeds the threshold of 5%, an indication for physiologically intact skin models, optimum culture conditions and highly reproducible tissue batches.